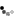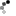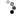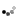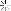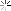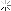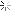Looky what we have here:
So I ground out the dull looking bits and filled it with an aluminum epoxy...
Any relevance to coolant choice at all?
Running 3-6 month old ethylene glycol at the moment. Flushed 20 gallons through the engine... ran it till the water came out just as clean as it went in.
Only thing GPF brought up was coolant filters.... Which I have to admit is a pretty neat concept.
Ford Mustang Tefba 1 1/4" Coolant Filter 64 1/2 - 73 - eBay (item 230524590357 end time Dec-09-10 07:46:15 PST)
Did some "research" and came up with this:
SpringerLink - Journal of Materials Science, Volume 42, Number 20
But that doesnt help me at all really... Also dont know if 3003 grade aluminum is even remotely close to what the S/C case is made of.Conclusions
The composition of the oxide film formed on aluminum
electrode in ethylene glycol–water solution depends on the
existence of dissolved oxygen. In the presence of oxygen, a
layer of aluminum oxide film forms on the aluminum
surface to protect the substrate from corrosion. In the absence
of oxygen, the film formed is mainly aluminumalcohol
film that is less compact and less resistant to corrosion.
The aluminum oxide film and aluminum-alcohol film
have the different susceptibilities to chloride ion attack for
pit initiation. There is a higher pitting susceptibility for
aluminum oxide-covered electrode.
The increase in temperature decreases the resistance of
aluminum oxide film to corrosion reaction. However, the
resistance to pitting corrosion increases.
According to this:
Aluminium alloy - Wikipedia, the free encyclopedia
3xxx's aren't used for ****... lol
So i'm left with this question...
What coolants (since no test will ever reveal what coolant is what..) would you recommend to help prevent pitting?
And yes the $8 1 gallon jugs of coolant i've been buying say right on the label: "Low silicates. Aluminum compatible"